

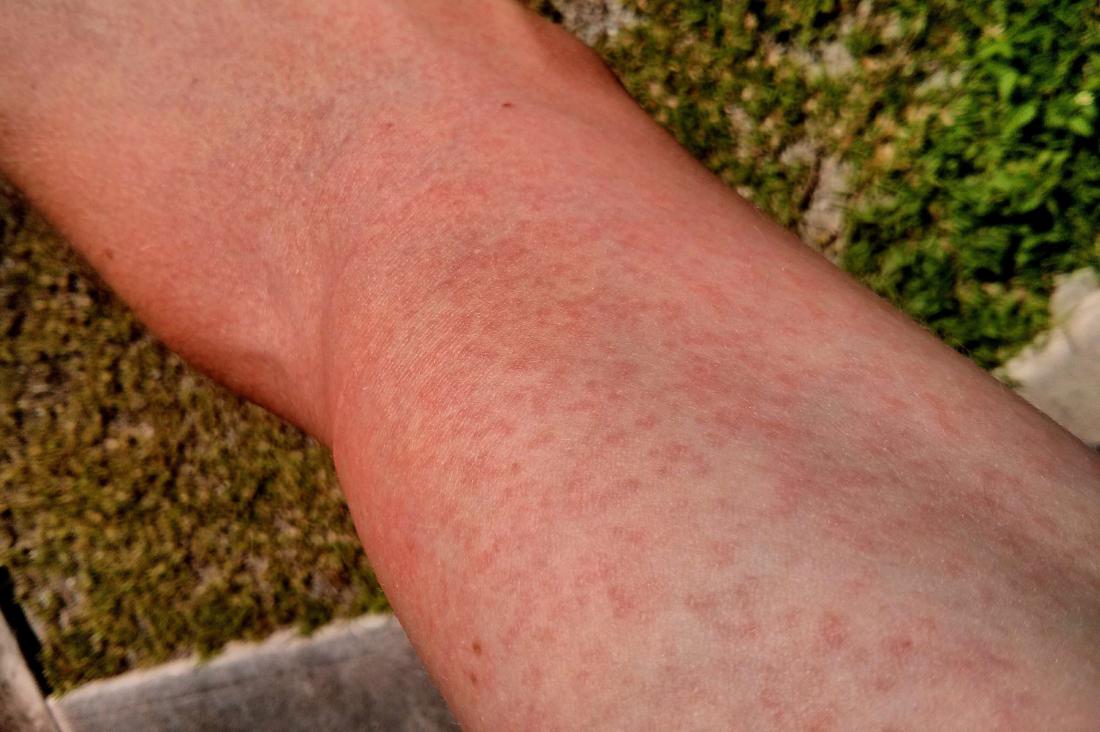
Although the management of rash caused by tkis and mAbs is clinically similar, there are differences between these two classes of egfr-targeted agents with regard to the incidence, severity, and onset of this skin toxicity.Ĭetuximab (Erbitux: Bristol–Myers Squibb, Princeton, NJ, U.S.A.) is a recombinant human/mouse (chimeric) immunoglobulin G1 mAb that is administered once weekly. The egfr-blocking tkis have been the subject of several publications on skin rash, and therefore the present article focuses on management of rash resulting from egfr-targeted mAbs for the treatment of gi malignancy. Inhibitors of this target can be broadly classified as either tyrosine kinase inhibitors ( tkis) or monoclonal antibodies (mAbs), both of which can produce significant skin toxicity. Thus, egfr is an ideal target for antitumour therapy. Dysregulated egfr may result in uncontrolled cell growth, proliferation, and angiogenesis, and is associated with a poorer prognosis, manifested by increased metastatic potential and poorer overall survival ( os) times 3.
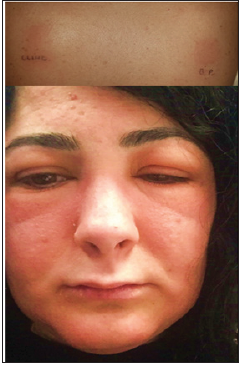
In many solid tumours, including most gastrointestinal ( gi) malignancies, egfr is overexpressed 2. The receptor consists of an extracellular ligand-binding domain, a transmembrane region, and an intracellular tyrosine kinase domain 1. The epidermal growth factor receptor ( egfr) is a trans-membrane glycoprotein that is expressed in many normal human cells of epithelial origin, playing an important role in cell growth, differentiation, and proliferation. Recommendations aimed at establishing a framework for consistent, proactive management of skin rash in the Canadian setting are presented. The present article focuses on egfr-targeted mAbs for the treatment of gi malignancy, addressing the pathophysiology, clinical presentation, and incidence of skin rash caused by this class of agents. In addition, effective management and patient education may help to alleviate the significant social and emotional anxiety related to this manageable side effect, thus resulting in improved quality of life. A proactive, multidisciplinary approach to management can help to improve skin rash and optimize clinical outcomes by preventing egfri dose reduction or discontinuation. This reversible condition requires intervention in approximately one third of patients. Although egfris have been proven effective in the treatment of a variety of malignancies, the entire class of agents is associated with a high prevalence of dermatologic side effects, most commonly skin rash. The egfr inhibitors ( egfris) approved in Canada include the tyrosine kinase inhibitors erlotinib and gefitinib (in selected cases), and the monoclonal antibodies (mAbs) panitumumab and cetuximab. Agents targeting the egfr-mediated signalling pathway are increasingly part of the therapeutic armamentarium for the treatment of advanced lung, head-and-neck, and colorectal carcinoma. The epidermal growth factor receptor ( egfr) is often overexpressed or dysregulated in a variety of solid tumours, including gastrointestinal ( gi) malignancies.


 0 kommentar(er)
0 kommentar(er)
